ORIGINAL ARTICLE
Stereocaulon tomentosoides, a new combination for
a western North American endemic species with cyanobiont
and chemotype polymorphisms
1
Department of Botany and Plant Pathology, Oregon State University,
Corvallis, Oregon 97331, USA
2
Czech Academy of Sciences, Institute of Botany, Zámek 1, 252 43
Průhonice, Czech Republic
3
Botany Unit, Finnish Museum of Natural History, P.O. Box 7,
FI-00014, University of Helsinki, Helsinki, Finland
Publication date: 2023-12-29
Plant and Fungal Systematics 2023; 68(2): 364-377
KEYWORDS
lichenslichenized fungiNorth AmericanuITS rDNAnuLSU rDNAStereocaulaceaeStereocaulon cyaneumStereocaulon sasakiiStereocaulon tomentosum
ABSTRACT
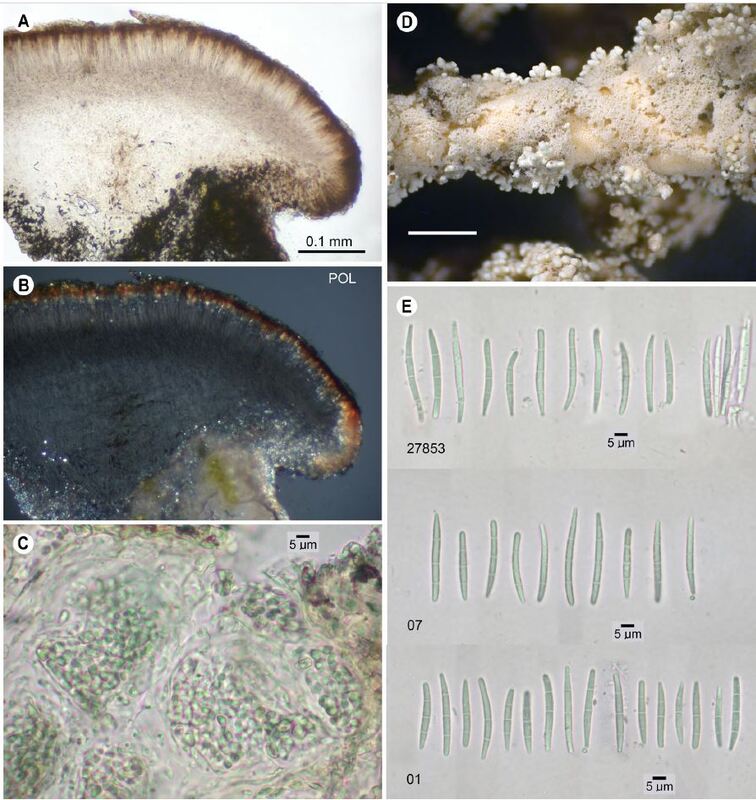
Based on resampling the type locality and surrounding regions, along with phylogenetic
analysis of molecular data, we elevate Stereocaulon sasakii var. tomentosoides to
the species level, while we treat S. sasakii var. simplex as an environmental modification of
S. tomentosoides. We found no phylogenetic evidence that any variety of S. sasakii occurs
in North America, so we suggest that the species be removed from the North American list
and its North American varieties transferred to S. tomentosoides. Stereocaulon tomentosoides
is so far confirmed only from the Pacific Northwest of North America. Furthermore, it
is largely allopatric with S. tomentosum, apart from a small region of overlap in northern
Idaho and western Montana. While S. tomentosum always contains stictic acid and never
lobaric acid as secondary metabolites, S. tomentosoides differs in having a predominant
chemotype of lobaric acid as the major substance, with an infrequent chemotype containing
both lobaric and stictic acids. While S. tomentosoides usually contains Nostoc in the cephalodia,
occasional individuals, especially from old mossy lava flows, contain Stigonema;
one specimen was found with both kinds of cephalodia on a single thallus. Phylogenetic
analysis of these species and other close relatives revealed an additional species described
here, S. cyaneum, so far known only from the Great Lakes region of the United States and
Canada and separated from S. tomentosum by its bluish coloration, wet or dry
FUNDING
NO
REFERENCES (22)
1.
Abadi, S., D. Azouri, Pupko, T. & Mayrose, I. 2019. Model selection may not be a mandatory step for phylogeny reconstruction. Nature Communications 10, 934: 1–10. https://doi.org/10.1038/s41467....
2.
Anisimova, M. & Gascuel, O. 2006. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Systematic Biology 55: 539–552. https://doi.org/10.1080/106351....
3.
Anisimova, M., Gil, M., Dufayard, J.-F., Dessimoz, C. & Gascuel, O. 2011. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Systematic Biology 60: 685–699. https://doi.org/10.1093/sysbio....
4.
Brodo, I. M., Sharnoff, S. D. & Sharnoff, S. 2001. Lichens of North America. Yale University Press, New Haven.
5.
Culberson, C. F. 1972. Improved conditions and new data for the identification of lichen products by a standardized thin-layer chromatographic method. Journal of Chromatography 72: 113–125. https://doi.org/10.1016/0021-9....
6.
Esslinger, T. L. 2019. A cumulative checklist for the lichen-forming, lichenicolous and allied fungi of the continental United States and Canada, Version 23. Opuscula Philolichenum 18: 102–378.
7.
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biology 59: 307–321. https://doi.org/10.1093/sysbio....
8.
Högnabba, F. 2006. Molecular phylogeny of the genus Stereocaulon (Stereocaulaceae, lichenized ascomycetes). Mycological Research 110: 1080−1092. https://doi.org/10.1016/j.mycr....
9.
Högnabba, F., Pino-Bodas, R., Nordin, A., Myllys, L. & Stenroos, S. 2014. Phylogenetic position of the crustose Stereocaulon species. The Lichenologist 46: 103−114. https://doi.org/10.1017/s00242....
10.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Mentjies, P. & Drummond, A. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. https://doi.org/10.1093/bioinf....
11.
Lamb, I. M. 1977. A conspectus of the lichen genus Stereocaulon (Schreb.) Hoffm. Journal of the Hattori Botanical Laboratory 43: 191–355.
12.
Lamb, I. M. 1978. Keys to the species of the lichen genus Stereocaulon (Schreb.) Hoffm. Journal of the Hattori Botanical Laboratory 44: 209–250.
13.
Mangold, A., Martín, M. P., Lücking, R. & Lumbsch, H. T. 2008. Molecular phylogeny suggests synonymy of Thelotremataceae within Graphidaceae (Ascomycota: Ostropales). Taxon 57: 476–486.
14.
McCune, B., Di Meglio, E., Tønsberg, T. & Yahr, R. 2019. Five new crustose Stereocaulon species in western North America. The Bryologist 122: 197–218. https://doi.org/10.1639/0007-2....
15.
McCune, B. & Geiser, L. 2009. Macrolichens of the Pacific Northwest. 2nd Edition. Oregon State University Press, Corvallis.
16.
Myllys, L., Högnabba, F., Lohtander, K., Thell, A., Stenroos, S. & Hyvönen, J. 2005. Phylogenetic relationships of Stereocaulaceae based on simultaneous analysis of beta-tubulin, GAPDH, and SSU rDNA sequences. Taxon 54: 605−618. https://doi.org/10.2307/250654....
17.
Park, J. S., Park, C.-H., Park, S.-Y., Oh, S.-O., Jayalal, U. & Hur, J.-S. 2018. Revision of the lichen genus Stereocaulon (Stereocaulaceae, Ascomycota) in South Korea. Mycobiology 46: 101–113. https://doi.org/10.1080/122980....
18.
Riddle, L. W. 1910. The North American Species of Stereocaulon. Botanical Gazette 50: 385–304. https://doi.org/10.1086/330363.
19.
Tønsberg, T. 1977. The chemical strains in Stereocaulon rivulorum and their distribution. Norwegian Journal of Botany 24: 231–234.
20.
Vančurová, L., Muggia, L., Peksa, O., Řídká, T., & Škaloud, P. 2018. The complexity of symbiotic interactions influences the ecological amplitude of the host: A case study in Stereocaulon (lichenized Ascomycota). Molecular Ecology 27: 3016–3033. https://doi.org/10.1111/mec.14....
21.
Vilgalys, R. & Hester, M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238−4246. https://doi.org/10.1128/jb.172....
22.
White, T. J., Bruns, T., Lee, S. H. & Taylor, J. 1990. 38 – Amplification and direct sequencing of fungal ribosomal DNA genes for phylogenetics. In: Innis, M. A., Gelfand, D. H., Sninsky, J. J. & White, T. J. (eds), PCR Protocols: A Guide to Methods and Applications, pp. 315–322. Academic Press, San Diego. https://doi.org/10.1016/b978-0....
CITATIONS (1):
1.
Crustose Stereocaulon species in Russia with a key to species
S. V. Chesnokov, L. A. Konoreva, A. S. Zueva
Novosti sistematiki nizshikh rastenii
S. V. Chesnokov, L. A. Konoreva, A. S. Zueva
Novosti sistematiki nizshikh rastenii
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.