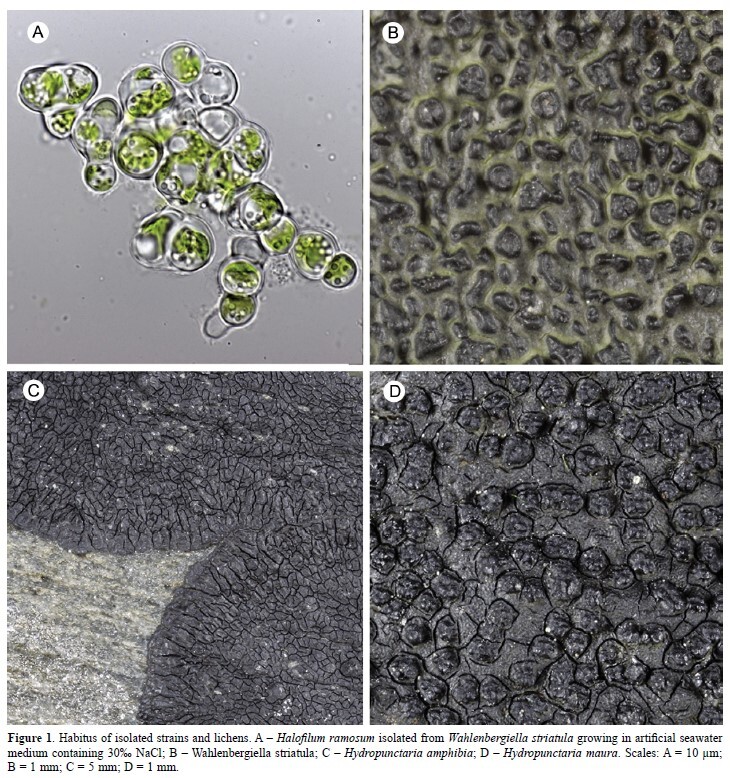
Differential responses to salt concentrations of lichen
photobiont strains isolated from lichens occurring in
different littoral zones
1
Departamento de Ciencias de la Vida, Universidad de Alcalá, Campus
Externo, 28805, Alcalá de Henares, Spain
2
Museo Nacional de Ciencias Naturales (CSIC), Calle Serrano 115-
dpdo, 28006, Madrid, Spain
3
Real Jardín Botánico (CSIC), Plaza Murillo 2, 28014 Madrid, Spain
Publication date: 2019-12-16
Plant and Fungal Systematics 2019; 64(2): 149-162
KEYWORDS
ABSTRACT
An interesting biota of lichen-forming fungi occurs along rocky seashores of cold
and warm-temperate regions in both hemispheres. Most of the species belong to the family
Verrucariaceae and form symbioses with an extraordinarily diverse group of photobionts.
We isolated the photobionts of three species: Hydropunctaria maura and H. amphibia from
the supralittoral zone, and Wahlenbergiella striatula from the upper intertidal zone. We
characterized the isolated strains structurally by means of transmission electron microscopy,
and molecularly using the nrSSU and nrITS and chloroplast RPL10A regions. Additionally,
we studied the response of the strains to different salt concentrations, analyzed the
concentration of osmoregulatory solutes, and measured photosynthesis performance by
chlorophyll fluorescence and CO2 assimilation techniques. All strains belong to the recently
described species Halofilum ramosum, although we found differences in the ITS and RPL10A
regions among the strains shared by H. maura and H. amphibia and the strain isolated
from W. striatula. Differences were also found in the main osmoregulatory response of the
strains growing under high salt concentrations: W. striatula accumulated glycerol, while
H. maura and H. amphibia synthetized sucrose. Analyses of photosynthesis performance
also indicated differences in physiological behavior between supralittoral-dwelling and
intertidal-dwelling species, W. striatula showing lower photosynthetic activity under high
irradiance. Our results highlight the role of photobionts in determining lichen zonation on
rocky seashores.
REFERENCES (83)
1.
Armstrong, R. A. & Smith, S. N. 1994. The levels of ribitol, arabitol and mannitol in individual lobes of the lichen Parmelia conspersa (Ehrh ex Ach) Ach. Environmental and Experimental Botany 34: 253–260.
2.
Batterton, J. & van Baalen, C. 1971. Growth Responses of Blue-Green Algae to Sodium Chloride Concentration. Archives Microbiology 76: 151–155.
3.
Ben-Amotz, A. & Avron, M. 1981. Glycerol and ß-carotene metabolism in the halotolerant alga Dunaliella: a model system for biosolar energy conversion. Trends in Biochemical Sciences 6: 297–299.
4.
Ben-Amotz, A. & Grunwald, T. 1981. Osmoregulation in the halotolerant alga Asteromonas gracilis. Plant Physiology 67: 613–616.
5.
Bilger, W. & Bjorkman, O. 1990. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbency changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynthesis Research 25: 173–185.
6.
Bisson, M. A. & Kirst, G. O. 1995. Osmotic acclimation and turgor pressure regulation in algae. Naturwissenschaften 82: 461–471.
7.
Bremauntz, M., Torres-Bustillos, L., Cañizares-Villanueva, R. & Fernández-Linares, L. 2011. Trehalose and sucrose osmolytes accumulated by algae as potential raw material for bioethanol. Natural Resources 2: 173–179.
8.
Brodo, I. M., & Santesson, R. 1997. Lichens of the Queen Charlotte Islands, British Columbia, Canada. 3. Marine species of Verrucaria (Verrucariaceae, Ascomycotina). The Journal of the Hattori Botanical Laboratory 82: 27–37.
9.
Brodo, I. M. & Sloan, N. A. 2004. Lichen zonation on coastal rocks in Gwaii Haanas National Park Reserve, Haida Gwaii (Queen Charlotte Islands), British Columbia. The Canadian Field-Naturalist 118: 405–424.
10.
Castresana, J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17: 540–552.
11.
Coste, C., Chauvet, E., Grieu, P. & Lamaze, T. 2016. Photosynthetic traits of freshwater lichens are consistent with the submersion conditions of their habitat. Annales de Limnologie – International Journal of Limnology 52: 235–242.
12.
Cowan, A. K., Rose, P. D. & Horne, L. G. 1992. Dunaliella salina – a model system for studying the response of plant-cells to stress. Journal Experimental Botany 43: 1535–1547.
13.
Darienko, T. & Pröschold, T. 2017. Toward a monograph of non-marine Ulvophyceae using an integrative approach (Molecular phylogeny and systematics of terrestrial Ulvophyceae II.). Phytotaxa 324: 1–41.
14.
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772.
15.
de los Ríos, A. & Ascaso, C. 2002. Preparative techniques for transmission electron microscopy and confocal laser scanning microscopy of lichens. In: Kranner, I., Beckett, R. P. & Varma, A. K. (eds), Protocols in Lichenology, pp. 87–117. Springer-Verlag, Berlin, Heidelberg.
16.
del Campo, E. M., Casano, L. M., & Barreno, E. 2013. Evolutionary implications of intron–exon distribution and the properties and sequences of the RPL10A gene in eukaryotes. Molecular Phylogenetics and Evolution 66: 857–867.
17.
Delmail, D., Grube, M., Parrot, D., Cook-Moreau, J., Boustie, J., Labrousse, P., & Tomasi, S. Halotolerance in Lichens: Symbiotic Coalition Against Salt Stress. In: Prasad, M. N. V., Azooz, M. M. & Parvaiz Ahmad (eds), Ecophysiology and Responses of Plants under Salt Stress, pp. 115–148. Springer-Verlag New York.
18.
Demmig-Adams, B. 1990. Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochimica et Biophysica Acta 1020: 1–24.
19.
Demmig-Adams, B. 1998. Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant and Cell Physiology 39: 474–482.
20.
Erdmann, N. & Hagemann, M. 2007. Salt acclimation of algae and cyanobacteria: a comparison. In: Rai, L. C. and Gaur, J. P. (eds) Algal adaptation to environmental stresses: physiological, biochemical and molecular mechanisms, pp. 322–360. Springer, Berlin.
21.
Fletcher, A. 1973a. The ecology of marine (littoral) lichens on some rocky shores of Anglesey. The Lichenologist 5: 368–400.
22.
Fletcher, A. 1973b. The ecology of maritime (supralittoral) lichens on some rocky shores of Anglesey. The Lichenologist 5: 401–422.
23.
Fletcher, A. 1980. Marine and maritime lichens of rocky shores: their ecology, physiology and biological interactions. In: Price, J. H., Irvine, D. E. G. & Farnham, W. F. (eds): The Shore Environment. Volume 2: Ecosystems, pp. 789–842. Academic Press, London & New York.
24.
Garrido-Benavent, I., Pérez-Ortega, S., & de los Ríos, A. 2017. From Alaska to Antarctica: species boundaries and genetic diversity of Prasiola (Trebouxiophyceae), a foliose chlorophyte associated with the bipolar lichen-forming fungus Mastodia tessellata. Molecular Phylogenetics and Evolution 107: 117–131.
25.
Genty B., Briantais J. M. & Baker N. R. 1989. The relationship between the quantum yield of photosynthetic electron-transport and quenching of Chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92.
26.
Goyal, A. 2007. Osmoregulation in Dunaliella, Part II: Photosynthesis and starch contribute carbon for glycerol synthesis during a salt stress in Dunaliella tertiolecta. Plant Physiology and Biochemistry 45: 705–710.
27.
Green, T. G. A., Büdel, B., Meyer, A., Zellner, H. & Lange, O. L. 1997. Temperate rainforest lichens in New Zealand: light response of photosynthesis. New Zealand Journal of Botany 35: 493–504.
28.
Green, T. G. A, Brabyn, L., Beard, C. & Sancho, L. G. 2012. Extremely low lichen growth rates in Taylor Valley, Dry Valleys, continental Antarctica. Polar Biology 35: 535–541.
29.
Gueidan, C., Roux, C. & Lutzoni, F. 2007. Using a multigene phylogenetic analysis to assess generic delineation and character evolution in Verrucariaceae (Verrucariales, Ascomycota). Mycological Research 111: 1145–1168.
30.
Gueidan, C., Savić, S., Thüs, H., Roux, C., Keller, C., Tibell, L., Prieto, M., Heiðmarsson, S., Breuss, O., Orange, A., Fröberg, L., Wynns, A. A., Navarro-Rosinés, P., Krzewicka, B., Pykälä, J., Grube, M. & Lutzoni, F. 2009. Generic classification of the Verrucariaceae (Ascomycota) based on molecular and morphological evidence: recent progress and remaining challenges. Taxon 58: 184–208.
31.
Hellebust J. A. 1985. Mechanisms of response to salinity in halotolerant microalgae. Plant and Soil 89: 69–81.
32.
Higgins, N. F., Connan, S., & Stengel, D. B. 2015. Factors influencing the distribution of coastal lichens Hydropunctaria maura and Wahlenbergiella mucosa. Marine Ecology 36: 1400–1414.
33.
Hill, D. J. & Ahmadjian, V. 1972. Relationship between carbohydrate movement and the symbiosis in lichens with green algae. Planta 103: 267–277.
34.
Hinton, R. H., Burge, M. L. E. & Hartman, G. C. 1969. Sucrose interference in the assay of enzymes and proteins. Analytical Biochemistry 29: 248–256.
35.
Joset, F., Jeanjean, R. & Hagemann, M. 1996. Dynamics of the response of cyanobacteria to salt-stress: Deciphering the molecular events. Physiologia Plantarum 96: 738–744.
36.
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A. & Jermiin, L. S. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14: 587–589.
37.
Katoh, K., Asimenos, G. & Toh, H. 2009. Multiple Alignment of DNA Sequences with MAFFT. In: Posada, D. (ed.). Bioinformatics for DNA Sequence Analysis, pp. 39–64. Totowa: Humana Press Inc.
38.
Kitajima, M. & Butler, W. L. 1975. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochimica et Biophysica Acta 376: 105–115.
40.
Lamb, I. M. 1973. Further observations on Verrucaria serpuloides M. Lamb, the only known permanently submerged marine lichen. Occasional papers of the Farlow Herbarium of Cryptogamic Botany 6: 1–5. .
41.
Loach, K. 1967. Shade tolerance in tree seedlings 1. Leaf photosynthesis and respiration in plants raised under artificial shade. New Phytologist 66: 607–621.
42.
Maphangwa, K. W., Musil, C. F., Raitt, L. & Zedda, L. 2012. Differential interception and evaporation of fog, dew and water vapour and elemental accumulation by lichens explain their relative abundance in a coastal desert. Journal of Arid Environments 82: 71–80.
43.
Meyer, M. T., Whittaker, C., Griffiths, H. 2017. The algal pyrenoid: key unanswered questions. Journal of Experimental Botany 68: 3739–3749.
44.
Minh, B. Q., Nguyen, M. A. T. & von Haeseler, A. 2013. Ultrafast Approximation for Phylogenetic Bootstrap. Molecular Biology and Evolution 30: 1188–1195.
45.
Moe, R. 1997. Verrucaria tavaresiae sp. nov., a marine lichen with a brown algal photobiont. Bulletin of the California Lichen Society 4: 7–11.
46.
Mohr, F., Ekman, S. & Heegaard, E. 2004.: Evolution and taxonomy of the marine Collemopsidium species (lichenized Ascomycota) in north-west Europe. Mycological Research 108: 515–532. .
47.
Nakayama, T., Watanabe, S., Mitsui, K., Uchida, H. & Inouye, I. 1996. The phylogenetic relationships between the Chlamydomonadales and Chlorococcales inferred from 18S rDNA sequence data. Phycological Research 44: 47–56.
49.
Nguyen, L. T., Schmidt, H. A., von Haeseler, A., & Minh, B. Q. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular biology and evolution 32: 268–274.
50.
Orange, A. 2012: Semi-cryptic marine species of Hydropunctaria (Verrucariaceae, lichenized Ascomycota) from north-west Europe. The Lichenologist 44: 299–320.
51.
Orange, A. 2013. British and other pyrenocarpous lichens. Version 2. Available at https://museum.wales/media/138....
52.
Oren, A. 2008. Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Systems 4: 2.
53.
Ortiz-Álvarez, R. de Los Ríos, A. Fernández-Mendoza, F., Torralba-Burrial, A. & Pérez-Ortega, S. 2015: Ecological specialization of two photobiont-specific maritime cyanolichen species of the genus Lichina. PLoS ONE 10: e0132718.
54.
Page-Sharp, M., Behm, C. & Smith, G. 1999. Involvement of compatible solutes trehalose and sucrose in the response to salt stress of cyanobaterial Scytonema species isolated from desert soils. 1472: 519–528.
55.
Papageorgiou, G. C. & Govindjee. 2014. The non-photochemical quenching of the electronically excited state of chlorophyll a in plants: Definitions, timelines, viewpoints, open questions. In: Demmig-Adams, B., Garab, G., Adams III, W., Govindjee (eds), Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, pp. 1–44. Springer Netherlands, Dordrecht.
56.
Parra, O. O. & Redon J. 1977. Aislamento de Heterococcus caespitosus Vischer ficobionte de Verrucaria maura Wahlenb. Boletín de la Sociedad de Biología de Concepción 51: 219–224.
57.
Pérez-Ortega, S., de los Ríos, A., Crespo, A. & Sancho, L. G. 2010. Symbiotic lifestyle and phylogenetic relationships of the bionts of Mastodia tessellata (Ascomycota, incertae sedis). American Journal of Botany 97: 738–752.
58.
Pérez-Ortega, S., Garrido-Benavent, I., Grube, M., Olmo, R. & de los Ríos, A. 2016. Hidden diversity of marine borderline lichens and a new order of fungi: Collemopsidiales (Dothideomyceta). Fungal Diversity 80: 285–300.
59.
Perez-Ortega, S., Miller, K. A. & de Los Rios, A. 2018. Challenging the lichen concept: Turgidosculum ulvae (Verrucariaceae) represents an independent photobiont shift to a multicellular blade-like alga. The Lichenologist 50: 341–356.
60.
Pick, U. 2002. Adaptation of the halotolerant alga Dunaliella to high salinity. In: Läuchli, A., Lüttge, U. (eds), Salinity: Environment – Plants – Molecules. Springer, Dordrecht .
61.
Picotto, M. & Tretiach, M. 2010. Photosynthesis in chlorolichens: the influence of the habitat light regime. Journal of Plant Research 123: 763–775.
62.
Piercey-Normore, M. D. & Depriest, P. T. 2001. Algal switching among lichen symbioses. American Journal of Botany 88: 1490–1498.
63.
Ramazanov, Z., Rawat, M., Henk, M. C., Matthews, S. W. & Moroney, J. V. (1994) The induction of the CO2-concentrating mechanism is correlated with the formation of the starch sheath around the pyrenoid of Chlamydomonas reinhardtii. Planta 195: 210–21.
64.
Reed, R. H., Richardson, D. L., Warr, S. R. & Stewart, W. D. 1984. Carbohydrate accumulation and osmotic stress in cyanobacteria. Journal of General Microbiology 130: 1–4.
65.
Reed, R. H. 1989. Osmotic adjustment and organic solute accumulation in Chaetomorpha capillaris. British Phycological Journal 24: 21–37.
66.
Renobales, G. & Noya, R. 1991. Estudio morfológico comparado de Verrucaria maura y V. amphibia en la Costa Vasca. Acta Botánica Malacitana 16: 149–156.
67.
Reynolds, E. S. 1963. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. Journal of Cell Biology 17: 208–212.
68.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A. & Huelsenbeck, J. P. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542.
69.
Roser, D. J., Melick, D. R., Ling, H. U. & Seppelt, R. D. 1992. Polyol and sugar content of terrestrial plants from continental Antarctica. Antartic Science 4: 413–420.
70.
Ryan, B. D. 1988. Zonation of lichens on a rocky seashore on Fidalgo Island, Washington. The Bryologist 91: 167–180.
71.
Sanders, W. B., Moe, R. L. & Ascaso, C. 2004. The intertidal marine lichen formed by the pyrenomycete fungus Verrucaria tavaresiae (Ascomycotina) and the brown alga Petroderma maculiforme (Phaeophyceae): thallus organization and symbiont interaction. American Journal of Botany 91: 511–522.
73.
Schreiber, U., Kühl, M., Klimant, I. &Reising, H. 1996. Measurement of chlorophyll fluorescence within leaves using a modified PAM fluorometer with a fiber-optic microprobe. Photosynthesis Research 47: 103–109.
74.
Schultz, M. 2017. Morphological and molecular data support Lichina intermedia as a distinct austral-marine species in the L. pygmaea group. The Lichenologist 49: 321–332. .
75.
Stacey, G., Van Baalen, C. &Tabita, F. 1977.Isolation and characterization of a marine Anabaena sp. capable of rapid growth on molecular nitrogen. Archives of Microbiology 144: 197–201.
76.
Taylor, R. M. 1982. Marine flora and fauna of the Northeastern United States. Lichens (Ascomycetes) of the intertidal region. NOAA Technical Report NMFS Circular 446, U.S. Department of Commerce, Washington.
77.
Thüs, H. & Schultz, M. 2008. Freshwater Flora of Central Europe, Vol. 21/1: Fungi, Part 1: Lichens. Heidelberg: Spektrum.
78.
Thüs, H., Muggia, L., Pérez-Ortega, S., Favero-Longo, S. E., Joneson, S., O’Brien, H., Nelsen, M. P., Duque-Thüs, R., Grube, M., Friedl, T., Brodie, J., Andrew, C. J., Lücking, R., Lutzoni, F. & Gueidan, C. 2011. Revisiting photobiont diversity in the lichen family Verrucariaceae (Ascomycota). European Journal of Phycology 46: 399–415.
79.
Trifinopoulos, J., Nguyen, L. T., von Haeseler, A., & Minh, B. Q. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44: W232–W235.
80.
van Kooten, O. & Snel, J. F. H. 1990. The use of chloroFhyl1 fluorescence nomenclature in plant stress physiology. Photosynthesis Research 25: 147–150.
81.
Warr, S. C. R., Reed, R. H. & Stewart, W. D. P. 1985. Carbohydrate accumulation in osmotically stressed cyanobacteria (Blue-Green Algae). New Phytologist 100: 285–292.
82.
White, T, J., Bruns, T. D., Lee, S. B. & Taylor, J. W. 1990. Amplification and direct sequencing of fungal ribosomal genes for phylogenies. In: Innis, M. A., Gelfand, D. H., Sninsky, J. J. & White, T. J. (eds), PCR protocols: a guide to methods and applications, pp. 315–322. Academic Press, San Diego.
83.
Zhan, Y., Marchand, C. H., Maes, A., Mauries, A., Sun, Y., Dhaliwal, J. S., Uniacke, J., Arragain, S., Jiang, H., Gold, N. D., Martin, V. J. , Lemaire, S. D., Zerges, W. 2018. Pyrenoid functions revealed by proteomics in Chlamydomonas reinhardtii. PLoS ONE 13: e0185039.
CITATIONS (17):
1.
Amphitropical variation of the algal partners of Pseudephebe (Parmeliaceae, lichenized fungi)
Isaac Garrido-Benavent, Sergio Pérez-Ortega, los de, Fernando Fernández-Mendoza
Symbiosis
Isaac Garrido-Benavent, Sergio Pérez-Ortega, los de, Fernando Fernández-Mendoza
Symbiosis
2.
Biocrust islands enhance infiltration, and reduce runoff and sediment yield on a heavily salinized dryland soil
Jalil Kakeh, Manouchehr Gorji, Mohammad Mohammadi, Hossein Asadi, Farhad Khormali, Mohammad Sohrabi, David Eldridge
Geoderma
Jalil Kakeh, Manouchehr Gorji, Mohammad Mohammadi, Hossein Asadi, Farhad Khormali, Mohammad Sohrabi, David Eldridge
Geoderma
4.
Polyols-related gene expression is affected by cyclic desiccation in lichen microalgae
Aline Hell, Francisco Gasulla, Maria González-Houcarde, Milena Pelegrino, Amedea Seabra, Campo del, Leonardo Casano, Danilo Centeno
Environmental and Experimental Botany
Aline Hell, Francisco Gasulla, Maria González-Houcarde, Milena Pelegrino, Amedea Seabra, Campo del, Leonardo Casano, Danilo Centeno
Environmental and Experimental Botany
6.
Complex photobiont diversity in the marine lichen Lichina pygmaea
Nathan Chrismas, Ro Allen, Anita Hollingsworth, Joe Taylor, Michael Cunliffe
Journal of the Marine Biological Association of the United Kingdom
Nathan Chrismas, Ro Allen, Anita Hollingsworth, Joe Taylor, Michael Cunliffe
Journal of the Marine Biological Association of the United Kingdom
7.
Lichen algae: the photosynthetic partners in lichen symbioses
William Sanders, Hiroshi Masumoto
The Lichenologist
William Sanders, Hiroshi Masumoto
The Lichenologist
8.
Lichens from the littoral zone host diverse Ulvophycean photobionts
Ivana Černajová, Ulf Schiefelbein, Pavel Škaloud, X. Johnson
Journal of Phycology
Ivana Černajová, Ulf Schiefelbein, Pavel Škaloud, X. Johnson
Journal of Phycology
9.
Genetic variation in the symbiont partners in the endangered macrolichen Seirophora villosa (Teloschistaceae: Ascomycota)
Isaac Garrido-Benavent, Arántzazu Molins, Eva Barreno
Botanical Journal of the Linnean Society
Isaac Garrido-Benavent, Arántzazu Molins, Eva Barreno
Botanical Journal of the Linnean Society
10.
Evaluation of the importance of ionic and osmotic components of salt stress on the photosynthetic efficiency of epiphytic lichens
Karolina Chowaniec, Kaja Rola
Physiology and Molecular Biology of Plants
Karolina Chowaniec, Kaja Rola
Physiology and Molecular Biology of Plants
11.
Combined effect of acute salt and nitrogen stress on the physiology of lichen symbiotic partners
Karolina Chowaniec, Anna Żukowska-Trebunia, Kaja Rola
Environmental Science and Pollution Research
Karolina Chowaniec, Anna Żukowska-Trebunia, Kaja Rola
Environmental Science and Pollution Research
12.
Does long-term salt stress of environmentally relevant concentrations affect the physiology of inland lichens? – The importance of rainfall to restore thallus vitality
Karolina Chowaniec, Ewa Latkowska, Kaja Rola
Environmental and Experimental Botany
Karolina Chowaniec, Ewa Latkowska, Kaja Rola
Environmental and Experimental Botany
13.
Lichen zonation on UK rocky seashores: a trait-based approach to delineating marine and maritime lichens
Beth Tindall-Jones, Michael Cunliffe, Nathan Chrismas
The Lichenologist
Beth Tindall-Jones, Michael Cunliffe, Nathan Chrismas
The Lichenologist
14.
Invariant properties of mycobiont‐photobiont networks in Antarctic lichens
Sergio Pérez‐Ortega, Miguel Verdú, Isaac Garrido‐Benavent, Sonia Rabasa, T. Green, Leopoldo Sancho, los de
Global Ecology and Biogeography
Sergio Pérez‐Ortega, Miguel Verdú, Isaac Garrido‐Benavent, Sonia Rabasa, T. Green, Leopoldo Sancho, los de
Global Ecology and Biogeography
15.
A synopsis of green-algal lichen symbionts with an emphasis on their free-living lifestyle
Veronika Veselá, Veronica Malavasi, Pavel Škaloud
Phycologia
Veronika Veselá, Veronica Malavasi, Pavel Škaloud
Phycologia
16.
The adaptation of lichen symbiosis to desert saline‐alkali stress depends more on their symbiotic algae
Biting Li, Reyim Mamuti, Liting Xiao, Ben Qian, Yanyan Wang, Xinli Wei
Physiologia Plantarum
Biting Li, Reyim Mamuti, Liting Xiao, Ben Qian, Yanyan Wang, Xinli Wei
Physiologia Plantarum
17.
The Biology, Microclimate, and Geology of a Distinctive Ecosystem Within the Sandstone of Hyper‐Arid Timna Valley, Israel
Irit Nir, Rachel Armoza‐Zvuloni, Hana Barak, Asunción De los Ríos, Christopher P. McKay, Ariel Kushmaro
Environmental Microbiology Reports
Irit Nir, Rachel Armoza‐Zvuloni, Hana Barak, Asunción De los Ríos, Christopher P. McKay, Ariel Kushmaro
Environmental Microbiology Reports
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.