ORIGINAL ARTICLE
Discovery of the first lichenized fungus in the family
Chaetothyriaceae (Ascomycota), Ceramothyrium ryukyuense
sp. nov.
1
Degree Programs in Life and Earth Sciences, Graduate School of Science
and Technology, University of Tsukuba, 1-1-1 Tennodai, Tsukuba,
Ibaraki, 305-8577, Japan
2
Department of Botany, National Museum of Nature and Science, 4-1-1
Amakubo, Tsukuba, 305-0005, Japan
3
School of Integrative and Global Majors, University of Tsukuba, 1-1-1
Tennodai, Tsukuba, Ibaraki, 305-8577, Japan
Online publication date: 2024-12-13
Publication date: 2024-12-13
Plant and Fungal Systematics 2024; 69(2): 167-176
KEYWORDS
ABSTRACT
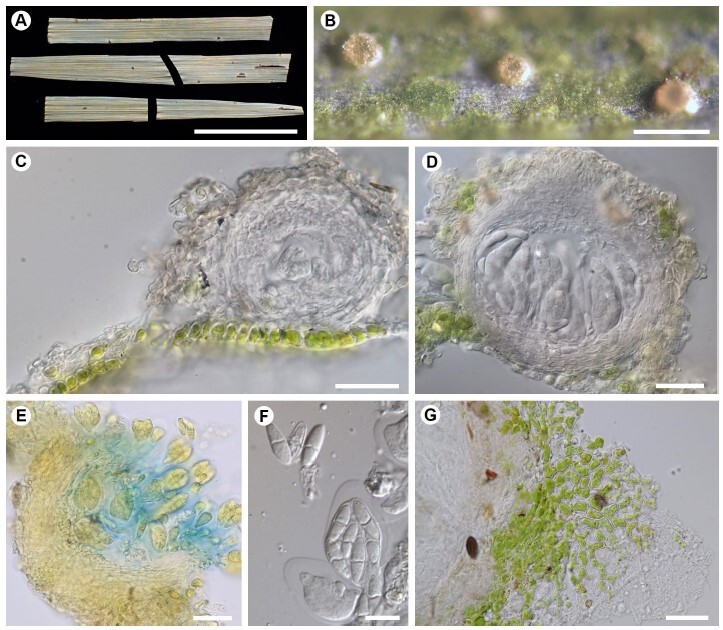
Ceramothyrium ryukyuense (Chaetothyriaceae) is described as a new species from
Okinawa, southern Japan. It is characterized by subglobose minute ascomata (up to 110 μm
diam.) covered with a brown mycelial pellicle, small ellipsoid 1(–2)-septate ascospores
(8.7–13.8 × 3.4–4.9 μm) within a small ascus (20–30 × 13–17 μm), and a lichenized thallus.
This species resembles non-lichenized Brazilian species, C. paiveae and C. philodendri, in
producing minute ascomata and 1–4 or 7 septate ascospores. However, besides its lichenized
status, C. ryukyuense is distinguished by its smaller asci (up to 30 μm long in C. ryukyuense
vs. 30–42 μm long in C. paiveae, and 50–100 μm long in C. philodendri), and predominantly
1-septate ascospores in C. ryukyuense, whereas multi-septate in C. paiveae and
C. philodendri. It was collected on a living leaf of Arecaceae in the subtropical forest near
the seashore. In a phylogenetic tree based on nuITS and nuLSU sequences, C. ryukyuense
formed a sister clade to Ceramothyrium exiguum which is known as an anamorphic species.
DNA sequences of C. paiveae and C. philodendri, morphologically similar species
to C. ryukyuense, were not available in this study. Algal cells distant from the perithecium
exhibited continuous branching, while those near the perithecium were strongly deformed
into a spherical shape and were partially unicellular. The photobiont of C. ryukyuense is
suggested to be a species of Trentepohliales, inferred from a phylogenetic analysis based
on the rbcl sequence. Ceramothyrium ryukyuense is the first report of a lichenized lineage
within Chaetothyriaceae.
FUNDING
This study
was partly supported by JSPS KAKENHI 22KJ0430 for the
first author.
REFERENCES (65)
1.
Asahina, Y. 1936. Mikrochemischer Nachweis der Flechtenstoffe (Ⅰ). Journal of Japanese Botany 12: 516–525.
2.
Barr, M. E. 1993. Redisposition of some taxa described by J.B. Ellis. Mycotaxon 46: 45–76.
3.
Batista, A. C. & Maia, H. S. 1956. Ceramothyrium, a new genus of the family Phaeosaccardinulaceae. Atti dell’Istituto Botanico e Laboratorio Crittogamico dell’Università di Pavia 14: 23–52.
4.
Batista, A. C. & Ciferri, R. 1962. The Chaetothyriales. Beihefte zur Sydowia 3: 1–129.
5.
Borgato, L., Ertz, D., Van Rossum, F. & Verbeken, A. 2022. The diversity of lichenized trentepohlioid algal (Ulvophyceae) communities is driven by fungal taxonomy and ecological factors. Journal of Phycology 58: 582–602. https://doi.org/10.1111/jpy.13....
6.
Chomnunti, P., Ko, T. W. K., Chukeatirote, E., Hyde, K. D., Cai, L., Jones, E. B. G., Kodsueb, R., Hassan, B. A. & Chen, H. 2012. Phylogeny of Chaetothyriaceae in northern Thailand including three new species. Mycologia 104: 382–395. https://doi.org/10.3852/11-066.
7.
Constantinescu, O., Holm, K. & Holm, L. 1989. Teleomorph-anamorph connections in Ascomycetes. 1–3. Stanhughesia (Hyphomycetes) new genus, the anamorph of Ceramothyrium. Studies in Mycology 31: 69–84.
8.
Crous, P. W., Schubert, K., Braun U., de Hoog, G. S., Hocking, A. D., Shin, H. D. & Groenewald, J. Z. 2007. Opportunistic, hu-man-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Ven-turiaceae. Studies in Mycology 58:185–217. https://doi.org/10.3114/sim.20....
9.
Crous, P. W., Braun, U., Wingfield, M. J., Wood, A. R., Shin, H. D., Summerell, B. A., Alfenas, A. C., Cumagun, C. J. & Groenewald, J. Z. 2009. Phylogeny and taxonomy of obscure genera of microfungi. Persoonia 22: 139–161. https://doi.org/10.3767/003158....
10.
Crous, P. W., Shivas, R. G., Wingfield, M. J., Summerell, B. A., Rossman, A. Y., Alves, J. L., Adams, G. C., Barreto, R. W., Bell, A. & Coutinho, M. L., et al. 2012. Fungal Planet description sheets: 128–153. Persoonia 29: 146–201. https://doi.org/10.3767/003158....
11.
Crous, P. W., Wingfield, M. J., Burgess, T. I., Hardy, G. E. St. J., Crane, C., Barrett, S., Cano-Lira, J. F., Le Roux, J. J., Thangavel, R. & Guarro, J., et al. 2016. Fungal Planet description sheets. 469–557 Persoonia 37: 218–403. https://doi.org/10.3767/003158....
12.
Crous, P. W., Luangsa-ard, J. J., Wingfield, M. J., Carnegie, A. J., Hernández-Restrepo, M., Lombard, L., Roux, J., Barreto, R. W., Baseia, I. G. & Cano-Lira, J. F., et al. 2018a. Fungal Planet description sheets: 785–867. Persoonia 41: 238–417. https://doi.org/10.3767/persoo....
13.
Crous, P. W., Wingfield, M. J., Burgess, T. I., Hardy, G. E. S. J, Gené, J., Guarro, J., Baseia, I. G., García, D., Gusmão, L. F. P. & Souza-Motta, C.M., et al. 2018b. Fungal Planet description sheets: 716–784. Persoonia 40: 240–393. https://doi.org/10.3767/persoo....
14.
Crous, P. W., Wingfield, M. J., Lombard, L., Roets, F., Swart, W. J., Alvarado, P., Carnegie, A. J., Moreno, G., Luangsa-Ard, J. & Thangavel, R., et al. 2019. Fungal Planet description sheets: 951–1041. Persoonia 43: 223–425. https://doi.org/10.3767/persoo....
15.
Culberson, C. F. & Johnson, A. 1982. Substitution of methyl tert.-butyl ether for diethyl ether in the standardized thin-layer chromatographic method for lichen products. Journal of Chromatography 238: 483–487. https://doi.org/10.1016/S0021-....
16.
Diederich, P., Palice, Z. & Ertz, D. 2008. Cheiromycina ananas is a synonym of Dictyocatenulata alba, a widespread, lichenized, synnematous hyphomycete herewith reported as new for Europe. Sauteria 15: 205–214.
17.
Frisch, A., Thor, G., Ertz, D. & Grube, M. 2014. The Arthonialean challenge: restructuring Arthoniaceae. Taxon 63: 727–744. https://doi.org/10.12705/634.2....
18.
Gardes, M. & Bruns, T. D. 1993. ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. https://doi.org/10.1111/j.1365....
20.
Honegger, R. 1986. Ultrastructural studies in lichens. I. Haustorial types and their frequencies in a range of lichens with trebouxioid photobionts. New Phytologist 103: 785–795. https://doi.org/10.1111/j.1469....
21.
Hongsanan, S., Hyde, K. D., Bahkali, A. H., Camporesi, E., Chomnunti, P., Ekanayaka, H., Gomes, A. A. M., Hofstetter, V., Jones, E. B. G. & Pinho, D. B.,Pereira, O. L., Tian, Q., Wanasinghe, D. N., Xu, J.-Ch. & Buyck, B. 2015. Fungal biodiversity profiles 11–20. Cryptogamie, Mycologie 36: 355–380. https://doi.org/10.7872/crym/v....
22.
Hyde, K. D., Hongsanan, S., Jeewon, R., Bhat, D. J., McKenzie, E. H. C., Jones, E. B. G., Phookamsak, R., Ariyawansa, H. A., Boonmee, S. & Zhao, Q., et al. 2016. Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 80: 1–270. https://doi.org/10.1007/s13225....
23.
Izumitsu, K., Hatoh, K., Sumita, T., Kitade, Y., Morita, A., Tanaka, Ch., Gafur, A., Ohta, A., Kawai, M., Yamanaka, T., Neda, H. & Ota, Y. 2012. Rapid and simple preparation of mushroom DNA directly from colonies and fruiting bodies for PCR. Mycoscience 53: 396–401. https://doi.org/10.1007/S10267....
24.
Kalb, K. & Vězda, A. 1994. Beiträge zur Kenntnis der foliicolen Flechten australischer Regenwälder IV. Bulletin de la Société linnéenne de Provence 45: 235–246.
25.
Katoh, K., Rozwicki, J. & Yamada, K. D. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20: 1160–1166. https://doi.org/10.1093/bib/bb....
26.
Kohlmeyer, J., Hawksworth, D. L. & Volkmann-Kohlmeyer, B. 2004. Observations on two marine and maritime “borderline” lichens: Mastodia tessellata and Collemopsidium pelvetiae. Mycological Progress 3: 51–56. https://doi.org/10.1007/s11557....
27.
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution 35: 1547–1549. https://doi.org/10.1093/molbev....
28.
Lambright, D. D., & Tucker, S. C. 1980. Observations on the ultrastructure of Trypethelium eluteriae Spreng. The Bryologist 83: 170–178.
29.
Liu, J. K., Hyde, K. D., Jones, E. B. G., Ariyawansa, H. A., Bhat, D. J., Boonmee, S., Maharachchikumbura, S. S. N., McKenzie, E. H. C., Phookamsak, R. & Phukhamsakda, C., et al. 2015. Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Diversity 72: 1–197. https://doi.org/10.1007/s13225....
30.
Lücking, R. 2008. Foliicolous lichenized Fungi. Flora Neotropica Monograph 103: 1–866.
31.
Lücking, R., Hodkinson, B. P. & Leavitt, S. D. 2017. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota – Approaching one thousand genera. The Bryologist 119: 361–416. https://doi.org/10.1639/0007-2....
32.
Maharachchikumbura, S. S. N., Haituk, S., Pakdeeniti, P., Al-Sadi, A. M., Hongsanan, S., Chomnunti, P. & Cheewangkoon, R. 2018. Phaeosaccardinula coffeicola and Trichomerium chiangmaiensis, two new species of Chaetothyriales (Eurotiomycetes) from Thailand. Mycosphere 9: 769–778. https://doi.org/10.5943/mycosp....
33.
Marasinghe, D. S., Hongsanan, S., Zeng, X. Y., Jones, E. B. G., Chomnunti, P., Boonmee, S. & Hyde, K. D. 2023. Taxonomic monograph of epifoliar fungi. Fungal Diversity 121: 139–334. https://doi.org/10.1007/s13225....
34.
Matthews, S. W., Tucker, S. C. & Chapman, R. L. 1989. Ultrastructural features of mycobionts and trentepohliaceous phycobionts in selected subtropical crustose lichens. Botanical Gazette 150: 417–438. https://doi.org/10.1086/337788.
35.
Meier, J. L. & Chapman, R. L. 1983. Ultrastructure of the lichen Coenogonium interplexum Nyl. American Journal of Botany 70: 400–407. https://doi.org/10.1002/j.1537....
36.
Miadlikowska, J. & Lutzoni, F. 2000. Phylogenetic revision of the genus Peltigera (lichen-forming Ascomycota) based on morphological, chemical, and large subunit nuclear ribosomal DNA data. International Journal of Plant Science 161: 925–958. https://doi.org/10.1086/317568.
37.
Miyazawa, K., Ohmura, Y. & Yamaoka, Y. 2022. Noteworthy foliicolous lichens collected from Iriomote Island, southern Japan. Taiwania 67: 155–163. https://doi.org/10.6165/tai.20....
38.
Morris, E. F. & Finley, D. D. 1967. Two new genera of stilbellaceous fungi. The American Midland Naturalist 77: 200–204.
39.
Nelsen, M. P., Rivas Plata, E., Andrew, C. J., Lücking, R. & Lumbsch, H. T. 2011. Phylogenetic diversity of trentepohlialean algae associated with lichen-forming fungi. Journal of Phycology 47: 282–290. https://doi.org/10.1111/j.1529....
40.
Ohmura, Y., Mizobuchi, A., Handa, S. & Lücking, R. 2016. Coenogonium moniliforme (Coenogoniaceae, Lichenized Ascomycota) new to Japan, with taxonomic notes of the photobiont in culture. Journal of Japanese Botany 91: 74–78.
41.
Ohmura, Y., Sugimoto, M., Aung, M. M. & Tanaka, N. 2020. Contribution to the knowledge of the lichen mycota of Myanmar (I) twenty species newly recorded from Southern Myanmar. Taiwania 65: 548–558. https://doi.org/10.6165/tai.20....
42.
Pérez-Ortega, S., Garrido-Benavent, I., Grube, M., Olmo, R. & de los Ríos, A. 2016. Hidden diversity of marine borderline lichens and a new order of fungi: Collemopsidiales (Dothideomyceta). Fungal Diversity 80: 285–300. https://doi.org/10.1007/s13225....
44.
Réblová, M, Untereiner, W. A. & Réblová, K. 2013. Novel evolutionary lineages revealed in the Chaetothyriales (fungi) based on multigene phylogenetic analyses and comparison of ITS secondary structure. PLoS ONE 8: e63547. https://doi.org/10.1371/journa....
45.
Rindi, F., Lam, D. W. & López-Bautista, J. M. 2009. Phylogenetic relationships and species circumscription in Trentepohlia and Printzina (Trentepohliales, Chlorophyta). Molecular phylogenetics and evolution 52: 329–339. https://doi.org/10.1016/j.ympe....
46.
Sanders, W., De Carolis, R., Ertz, D., de los Ríos, A. & Muggia, L. 2023. Independent, structurally distinct transitions to microfruticose growth in the crustose genus Porina (Ostropales, Lecanoromycetes): new isidioid species from south-western Florida. The Lichenologist 55: 347–365. https://doi.org/10.1017/S00242....
47.
Santesson, R. 1952. Foliicolous lichens I. A revision of the taxonomy of the obligately foliicolous, lichenized fungi. Symbolae Botanicae Upsalienses 12: 1–590.
48.
Schumm, F. & Elix, J.A. 2015. Atlas of Images of Thin Layer Chromatograms of Lichen Substances. Books on Demand GmbH, Norderstedt. 584 pp.
49.
Takeshita, S., Nakanishi, M. & Akiyama, M. 1999. Morphological variations of the photobionts isolated from the lichen family Graphidaceae. Bulletin of the Graduate School of Education, Hiroshima University, Part. Ⅱ 21: 43–47 (in Japanese).
50.
Tamura, K. 1992. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Molecular Biology and Evolution 9: 678–687. https://doi.org/10.1093/oxford....
51.
Tamura, K. & Nei, M. 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10: 512–526.
52.
Tennakoon, D. S., Thambugala, K. M., Jeewon, R., Hongsanan, S., Kuo, C. H. & Hyde, K. D. 2019. Additions to Chaetothyriaceae (Chaetothyriales): Longihyalospora gen. nov. and Ceramothyrium longivolcaniforme, a new host record from decaying leaves of Ficus ampelas. MycoKeys 61: 91–109. https://doi.org/10.3897/mycoke....
53.
Thor, G., Lücking, R. & Matsumoto, T. 2000. The foliicolous lichens of Japan. Symbolae Botanicae Upsalienses 32: 1–72.
54.
Tucker, S. C., Matthews, S. W. & Chapman, R. L. 1991. Ultrastructure of subtropical crustose lichens. In: Galloway, D. J. (ed.), Tropical Lichens: Their Systematics, Conservation and Ecology, pp. 171–191. Clarendon Press, Oxford.
55.
Vilgalys, R. & Hester, M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. https://doi.org/10.1128/jb.172....
56.
Vu, D., Groenewald, M., de Vries, M., Gehrmann, T., Stielow, B., Eberhardt, U., Al-Hatmi, A., Groenewald, J. Z., Cardinali, G., Houbraken, J., Boekhout, T., Crous, P. W., Robert, V., & Verkley, G. J. M. 2019. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. https://doi.org/10.1016/j.simy....
57.
Wang, W. C. & Wei, J. C. 2018. Arthonia, Byssoloma, Calenia, Chroodiscus, Coenogonium, Eremothecella, and Semigyalecta spp. new to China. Mycotaxon 133: 487– 497. https://doi.org/10.5248/133.48....
58.
Wang, W. C., Sangvichien, E., Wei, T. Z. & Wei, J. C. 2020. A molecular phylogeny of Pilocarpaceae Zahlbr., including a new species of Tapellaria Müll. Arg. and new records of foliicolous lichenized fungi from Thailand. The Lichenologist 52: 377–385. https://doi.org/10.1017/S00242....
59.
Wedin, M., Döring, H. & Gilenstam, G. 2004. Saprotrophy and lichenization as options for the same fungal species on different substrata: environmental plasticity and fungal lifestyles in the Stictis-Conotrema complex. New Phytologist 164: 459– 465. https://doi.org/10.1111/j.1469....
60.
Wijesinghe, S. N., Dayarathne, M. C., Maharachchikumbura, S. S. N., Wanasinghe, D. N. & Hyde, K. D. 2019. Ceramothyrium chiangraiense, a novel species of Chaetothyriales (Eurotiomycetes) from Ficus sp. in Thailand. Asian Journal of Mycology 2: 269–280. https://doi.org/10.5943/ajom/2....
61.
Yang, H., Chomnumti, P., Aryawansa, H., Wu, H. X. & Hyde, K. D. 2014. The genus Phaeosaccardinula (Chaetothyriales) from Yunnan, China, introducing two new species. 2014. Chiang Mai Journal of Science 41: 873–884.
62.
Yang, H., Hyde, K. D., Karunarathna, S. C., Deng, C., Gu, C. H., Yang, S. A. & Zhang, Z. C. 2018. New species of Camptophora and Cyphellophora from China, and first report of sexual morphs for these genera. Phytotaxa 342: 149–159. https://doi.org/10.11646/phyto....
63.
Yen, L. T. H., Tsurumi, Y., Hop, V. D. & Ando, K. 2018. Three new anamorph of Ceramothyrium from fallen leaves in Vietnam. Advances in Microbiology 8: 314–323. https://doi.org/10.4236/aim.20....
64.
Zeng, X. Y., Wen, T. C., Chomnunti, P., Liu, J. K., Boonmee, S. & Hyde, K. D. 2016. Ceramothyrium longivolcaniforme sp. nov., a new species of Chaetothyriaceae from northern Thailand. Phytotaxa 267: 51–60. https://doi.org/10.11646/phyto....
65.
Zoller, S., Scheidegger, C. & Sperisen, C. 1999. PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming Ascomycetes. The Lichenologist 31: 511–516. https://doi.org/10.1006/lich.1....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.