ORIGINAL ARTICLE
The morphology, phylogeny, and distribution of the pine-associated sequestrate fungus Lactarius cinnabarinus comb. nov. (= Zelleromyces cinnabarinus) in the United States, Argentina and Brazil
1
Instituto de Botánica del Nordeste (IBONE-UNNE-CONICET), Sargento
Cabral 2131, 3400, Corrientes, Argentina
2
University of Florida, Department of Plant Pathology, Florida, USA
3
Universidad Nacional del Nordeste, Facultad de Ciencias Exactas y
Naturales y Agrimensura (FaCENA-UNNE), Corrientes, Argentina
4
IFungiLab, Federal Institute of Education, Science and Technology of
São Paulo (IFSP), Campus São Paulo (SPO), Department of Natural
Sciences and Mathematics (DCM), São Paulo, Brazil
Online publication date: 2024-11-29
Publication date: 2024-11-29
Plant and Fungal Systematics 2024; 69(2): 125-134
KEYWORDS
ABSTRACT
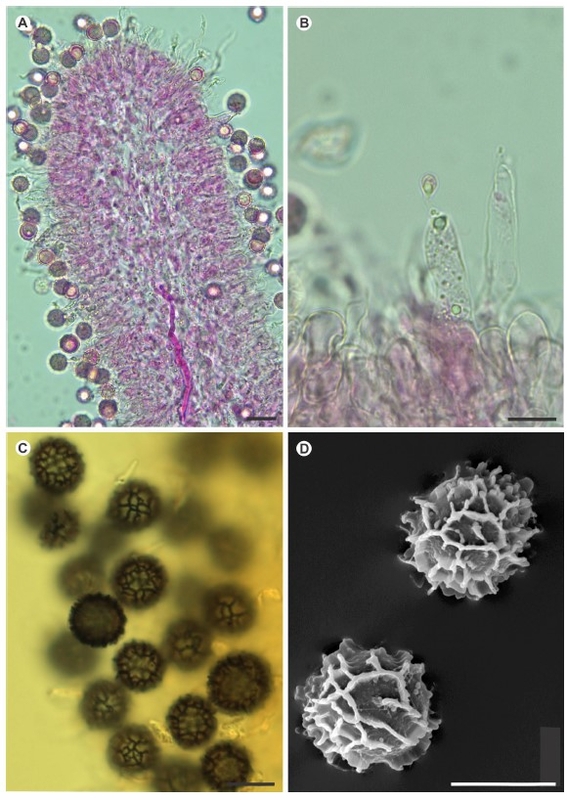
The sequestrate fungus Zelleromyces cinnabarinus was originally found beneath
pines and was described from Louisiana, USA. Here we re-evaluate the morphology, taxonomy,
and distribution of this species based on new specimens across the native range in
the southeastern United States (USA), as well as introduced populations in Argentina and
Brazil. Internal Transcribed Spacer (ITS) sequences of fungarium specimens indicate that
this species is widespread in the southeastern USA with native pines and has been introduced
to South America in cultivated pine plantations. An environmental sequence from
ectomycorrhizal Pinus roots also confirms the symbiotic association with pines. Our data
also indicate that Lactarius taedae, a sequestrate fungus recently described from Brazil,
is a later synonym of Zelleromyces cinnabarinus. Lastly, our phylogenetic analysis of ITS
sequences suggests that Zelleromyces cinnabarinus is nested within the genus Lactarius,
so we recombine this sequestrate species as Lactarius cinnabarinus comb. nov.
FUNDING
In Argentina, this work was supported by the Secretaría General
de Ciencia y Técnica, Universidad Nacional del Nordeste (SGCyT-
UNNE), Agencia de Promoción Científica y Tecnológica
(FONCYT), and Consejo Nacional de Investigaciones Científicas
y Tecnológicas (CONICET). Participation by M.E. Smith
and B. Lemmond was supported by the US National Science
foundation grant (DEB-2106130 to MES) and by the USDA
National Institute of Food and Agriculture, McIntire-Stennis
project FLA-PLP-006168 (to MES) AGS. Silva-Filho thanks
the ‘Fundação de Amparo a Pesquisa do Estado de São Paulo’
(Fapesp grant #2021/09109-1) for financial support.
REFERENCES (50)
1.
Barge, E. G., Cripps, C. L. & Osmundson, T. W. 2016. Systematics of the ectomycorrhizal genus Lactarius in the Rocky Mountain alpine zone. Mycologia 108(2): 414–440. https://doi.org/10.3852/15-177.
2.
Buyck, B., Hofstetter, V., Eberhardt, U., Verbeken, A. & Kauff, F. 2008. Walking the thin line between Russula and Lactarius: the dilemma of Russula subsect. Ochricompactae. Fungal Diversity 28: 15–40.
3.
Calonge, F. D. & Martín, M. P. 2000. Morphological and molecular data on the taxonomy of Gymnomyces, Martellia and Zelleromyces (Russulales). Mycotaxon 76: 9–15.
4.
Chen, Y. 2013. Ectomycorrhizal Fungal Diversity of Abies kawakamii and Tsuga chinensis var. formosana in a Subalpine Forest of Taiwan Determined by Molecular Analysis. Master’s Thesis. Tunghai University, Department of Life Sciences. 122 p.
5.
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. https://doi.org/10.1038/nmeth.....
6.
Desjardin, D. E. 2003. A unique ballistosporic hypogeous sequestrate Lactarius from California. Mycologia 95(1): 148–155.
7.
Eberhardt, U. & Verbeken, A. 2004. Sequestrate Lactarius species from tropical Africa: L. angiocarpus sp. nov. and L. dolichocaulis comb. nov. Mycological Research 108(9): 1042–1052. https://doi.org/10.1017/S09537....
8.
Edgar, R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32(5): 1792–1797. https://doi.org/10.1093/nar/gk....
9.
Gardes, M. & Bruns, T. D. 1993. ITS primers with enhanced specificity of basidiomycetes: Application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. https://doi.org/10.1111/j.1365....
10.
Guindon, S. & Gascuel, O. 2003. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. https://doi.org/10.1080/106351....
11.
Hampe, K. D. H. & Verbeken, A. 2015. Lactarius subgenus Russularia (Russulaceae) in South-East Asia: 3. new diversity in Thailand and Vietnam. Phytotaxa 207(3): 215–241. https://doi.org/10.1111/j.1471....
12.
Horton, B. M. 2011. Eucalypt decline and ectomycorrhizal fungal community ecology of Eucalyptus delegatensis forest, Tasmania, Australia. [PhD thesis, University of Tasmania], from <https://eprints.utas.edu.au/12...>.
13.
Index Fungorum – Authors of Fungal Names. 2023. Retrieved February, 14, 2023, from <http://www.indexfungorum.org/n...>.
14.
Ivanova, N. V., Fazekas, A. J. & Hebert, P. D. N. 2008. Semiautomated, membrane-based protocol for DNA isolation from plants. Plant Molecular Biology Reporter 26: 186–198. https://doi.org/10.1007/s11105....
15.
Ivanova, N. V., Dewaard, J. & Hebert, P. D. N. 2006. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Molecular Ecology Notes 6: 998–1002. https://doi.org/10.1111/j.1471....
16.
Ivanova, N. V., Kuzmina, M. & Fazekas, A. 2016. CCDB Protocols. Glass Fiber Plate DNA Extraction Protocol for plants, fungi, echinoderms and mollusks, Manual protocol employing centrifugation. Retrieved February, 14, 2023, from <http://ccdb.ca/site/wp-content...>.
17.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Mentjies, P. & Drummond, A. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647–1649. https://doi.org/10.1093/bioinf....
18.
Kornerup, A. & Wanscher, J. H. 1978. Methuen handbook of colour. Eyre Methuen Publishers, London. 252 pp.
19.
Kuhar, F., Nouhra, E., Pfister, D. H. & Smith, M. E. 2023. Paedomorphosis and evolution of sequestrate Basidiomycetes. In: Pöggeler, S., James, T. (eds) Evolution of Fungi and Fungal-Like Organisms. The Mycota, vol 14, pp. 295–314. Cham: Springer International Publishing. https://doi.org/10.1007/978-3-....
20.
Lebel, T. 2001. A new species of Zelleromyces (Russulales) from Australia. Australasian Mycologist 20(2): 4–8.
21.
Lebel, T. & Tonkin, J. E. 2007. Australasian species of Macowanites are sequestrate species of Russula (Russulaceae, Basidiomycota). Australian Systematic Botany 20(4): 355–381. https://doi.org/10.1071/SB0700....
22.
Lebel, T., Cooper, J. A., Castellano, M. A. & Nuytinck, J. 2021. Three independent evolutionary events of sequestrate Lactifluus species in Australasia. Fungal Systematics and Evolution 8(1): 9–25. https://doi.org/10.3114/fuse.2....
23.
Lee, H., Wissitrassameewong, K., Park, M. S., Verbeken, A., Eimes, J. & Lim, Y. W. 2019. Taxonomic revision of the genus Lactarius (Russulales, Basidiomycota) in Korea. Fungal Diversity 95(1): 275–335. https://doi.org/10.1007/s13225....
24.
Looney, B. P., Meidl, P., Piatek, M. J., Miettinen, O., Martin, F. M., Matheny, P. B. & Labbé, J. L. 2018. Russulaceae: a new genomic dataset to study ecosystem function and evolutionary diversification of ectomycorrhizal fungi with their tree associates. New Phytologist 218(1): 54–65. https://doi.org/10.1111/nph.15....
25.
Miller, M. A., Pfeiffer, W. & Schwartz, T. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. In: SC10 Workshop on Gateway Computing Environments (GCE10). Ieee 1–8. https://doi.org/10.1109/GCE.20....
26.
Molina, R., Pilz, D., Smith, J., Dunham, S., Dreisbach, T., O’Dell, T. & Castellano, M. 2001. Conservation and management of forest fungi in the Pacific Northwestern United States: an integrated ecosystem approach. In: Moore, D., Nauta, M. M., Evans, S. E. & Rotheroe, M. (eds) Fungal conservation: issues and solutions. Cambridge University Press 22: 19–63. https://doi.org/10.1017/CBO978....
27.
Montoya, L., Haug, I. & Bandala, V. M. 2010. Two Lactarius species associated with a relict Fagus grandifolia var. mexicana population in a Mexican montane cloud forest. Mycologia 102(1): 153–162. https://doi.org/10.3852/09-010.
28.
MyCoPortal. (n. d.). Map Search. Retrieved March 13, 2023, from <http://www.mycoportal.org/port...>.
29.
Nuytinck, J., Verbeken, A., Delarue, S. & Walleyn, R. 2003. Systematics of European sequestrate lactarioid Russulaceae with spiny spore ornamentation. Belgian Journal of Botany 136(2): 145–153.
30.
Osmundson, T. W., Robert, V. A., Schoch, C. L., Baker, L. J., Smith, A., Robich, G., Mizzan, L. & Garbelotto, M. M. 2013. Filling gaps in biodiversity knowledge for macrofungi: contributions and assessment of an herbarium collection DNA barcode sequencing project. Plos One 8(4): e62419. https://doi.org/10.1371/journa....
31.
Palmer, J. M., Lindner, D. L. & Volk, T. J. 2008. Ectomycorrhizal characterization of an American chestnut (Castanea dentata)-dominated community in Western Wisconsin. Mycorrhiza 19: 27–36. https://doi.org/10.1007/s00572....
32.
Pierotti, A. 2015. Nomenclatural novelties: Lactarius spp. Index Fungorum 254: 1.
33.
Põlme, S., Bahram, M., Yamanaka, T., Nara, K., Dai, Y. C., Grebenc, T., Kraigher, H., Toivonen, M., Wang, P.-H., Matsuda, Y., Naadel, T., Kennedy, P. G., Kõljalg, U. & Tedersoo, L. 2013. Biogeography of ectomycorrhizal fungi associated with alders (Alnus spp.) in relation to biotic and abiotic variables at the global scale. New Phytologist 198: 1239–1249. https://doi.org/10.1111/nph.12....
34.
Rennick, B., Benucci, G. M. N., Du, Z. Y., Healy, R. & Bonito, G. 2023. Tuber rugosum, a new species from northeastern North America: slug mycophagy aides in electron microscopy of ascospores. Mycologia 115: 340–356. https://doi.org/10.1080/002755....
35.
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. 2012. NIH Image to Image J: 25 years of image analysis. Nature Methods 9: 671–675. https://doi.org/10.1038/nmeth.....
36.
Schoch, C. L., Seifert, K. A., Huhndorf, S., Robert, V., Spouge, J. L., Levesque, C. A., ... & White, M. M. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences 109(16): 6241–6246. https://doi.org/10.1073/pnas.1....
37.
Silva-Filho, A. G. S., Sulzbacher, M. A., Ferreira, R. J., Baseia, I. G. & Wartchow, F. 2018. Lactarius taedae (Russulales): an unexpected new gasteroid fungus from Brazil. Phytotaxa 379(3): 234–246. https://doi.org/10.11646/phyto....
38.
Singer, R. & Smith, A. H. 1960. Studies on secotiaceous fungi. IX. The astrogastraceous series. Memoirs of the Torrey Botanical Club 21(3): 1–112.
39.
Stamatakis, A. 2014. RAxML version 8: a tool forphylogenetic analysis post-analysis of large phylogenies. Bioinformatics 30(9): 1312– 1313. https://doi.org/10.1093/bioinf....
40.
Sundberg, W. J. & Trappe, J. M. 1975. Notes on Zelleromyces cinnabarinus (Basidiomycetes, Hydnangiales). Mycologia 67(6): 1176–1181. https://doi.org/10.1080/002755....
41.
Talavera, G. & Castresana, J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from sequence alignments. Systematic Biology 56(4): 564–577. https://doi.org/10.1080/106351....
42.
Thiers, B. [continuously updated] (2023) Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Retrieved February, 14, 2023, from <http://sweetgum.nybg.org/ih/>.
43.
Ulyshen, M. D., Shefferson, R., Horn, S., Taylor, M. K., Bush, B., Brownie, C., Seibold, S. & Strickland, M. S. 2017. Below‐ and above‐ground effects of deadwood and termites in plantation forests. Ecosphere 8(8): e01910. https://doi.org/10.1002/ecs2.1....
44.
Verbeken, A., Stubbe, D., Van de Putte, K., Eberhardt, U. & Nuytinck, J. 2014. Tales of the unexpected: angiocarpous representatives of the Russulaceae in tropical South East Asia. Persoonia 32(1): 13–24. https://doi.org/10.3767/003158....
45.
Vidal, J. M., Alvarado, P., Loizides, M., Konstantinidis, G., Chachuła, P., Mleczko, P., Moreno, G., Vizzini, A., Krakhmalnyi, M., Paz, A. & Cabero, J. 2019. A phylogenetic and taxonomic revision of sequestrate Russulaceae in Mediterranean and temperate Europe. Persoonia 42(1): 127–185. https://doi.org/10.3767/persoo....
46.
Wang, R., Herrera, M., Xu, W., Zhang, P., Moreno, J. P., Colinas, C. & Yu, F. 2022. Ethnomycological study on wild mushrooms in Pu’er Prefecture, Southwest Yunnan, China. Journal of Ethnobiology and Ethnomedicine 18(1): 55. https://doi.org/10.1186/s13002- 022-00551-7.
47.
Warmink, J. A., Nazir, R. & Van Elsas, J. D. 2009. Universal and species‐ specific bacterial ‘fungiphiles’ in the mycospheres of different basidiomycetous fungi. Environmental Microbiology 11(2): 300–312. https://doi.org/10.1111/j.1462....
48.
Wisitrassameewong, K., Looney, B. P., Le, H. T., De Crop, E., Das, K., Van de Putte, K., Eberhardt, U., Jiayu, G., Stubbe, D., Hyde, K. D. & Verbeken, A. 2016. Lactarius subgenus Russularia (Basidiomycota, Russulales): novel Asian species, worldwide phylogeny and evolutionary relationships. Fungal Biology 120(12): 1554–1581. https://doi.org/10.1016/j.funb....
49.
Wisitrassameewong, K., Nuytinck, J., Hyde, D. & Verbeken, A. 2014. Lactarius subgenus Russularia (Russulaceae) in Southeast Asia: 1. Species with very distant gills. Phytotaxa 158(1): 23–42. https://doi.org/10.11646/phyto....
50.
Wisitrassameewong, K., Nuytinck, J., Thanh Le, H., De Crop, E., Hampe, F., Hyde, K.D. & Verbeken, A. 2015. Lactarius subgenus Russularia (Russulaceae) in South-East Asia: 3. new diversity in Thailand and Vietnam. Phytotaxa 207(3): 215–241. https://doi.org/10.11646/phyto....
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.